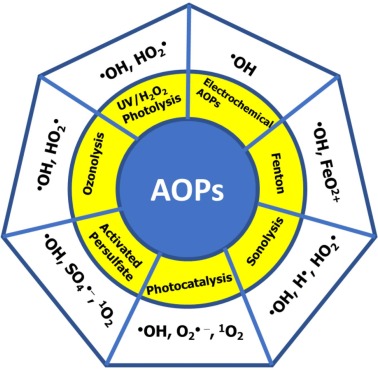
Advanced Oxidation Processes (AOPs) are defined as oxidation processes that use OH* free radicals as oxidizing agents, the corresponding techniques are called advanced oxidation techniques. AOTs). The AOP processes differ in that they generate different OH* radicals.
To treat toxic or non-biodegradable organic matter with not too high concentrations in wastewater, chemical oxidation methods can be used.
In fact, many substances that are difficult to biodegrade are also difficult to oxidize, so strong oxidizing agents are required.
The oxidizing capacity of oxidizing agents is assessed through the redox potential, the higher the redox potential, the stronger the oxidizing agent.
Among the common oxidizing agents, the OH* radical is the strongest, only inferior to fluorine.

Free radicals *OH (hydroxyl) are very active and have the ability to decompose substances with stable structures.
For example: benzene, colored complex groups, etc. These organic groups have a very stable structure and are difficult to decompose. The presence of these compounds in wastewater causes serious environmental pollution, affects ecosystems, aquatic plants and animals, and may be carcinogenic to humans.
In addition to the oxidation potential, the reaction rate must also be taken into account. The reaction rates are compared through the rate constant k. The k constant of the reactions between OH* radicals with organic substances is very high, millions to billions of times higher than that of ozone.
GENERAL CHARACTERISTICS OF HIGH-ORDER OXIDATION PROCESSES
- Combined with Hydroxyl
R + OH* -> ROH*
The above addition reaction occurs at a fast rate.
Hydroxyl radicals can cleave the hydrogen atom of an organic compound to produce an active organic radical:
Rn + OH* -> R* + H2O
- Electronic teleportation
The process of electron transfer will create new ions with higher valence.
Rn + OH* -> Rn-1 + OH-
The reaction process continues to develop thanks to new free radicals generated in a chain reaction fashion until complete mineralization (mineralization) or the chain reaction is broken.
- Free radical recombination
Two free radicals can combine to form a stable compound:
OH* + OH* -> H2O2
ROLE OF AOP IN WASTE WATER TREATMENT
AOP is capable of cleaning a wide range of industrial wastewater containing non-biodegradable or non-biodegradable organic compounds such as aromatics, pesticides, petroleum components and volatile organic compounds. a little bit. Therefore, AOP can be applied to the secondary treatment stage (before the biological tank) or the tertiary treatment stage (after settling, filtering).
The ultimate goal of higher oxidation processes is to “mineralize” the pollutants in the wastewater, that is, to convert organic pollutants into simple and non-toxic “inorganic” substances. Detail:
- Carbon in the pollutant molecule to carbon dioxide
- Hydrogen in the pollutant molecule turns into water
- Phosphorus in the pollutant molecule to phosphate or phosphoric acid
- Sulfur in the pollutant molecule to sulfate Nitrogen in the pollutant molecule to nitrate
- Halogen in the pollutant molecule to halogenic acid
- Inorganic compounds form higher oxidation states such as Fe2+ to Fe3+
When applied under properly regulated conditions, AOP can reduce contaminant concentrations from a few hundred ppm to less than 5ppm, thereby significantly reducing COD and TOC.
MECHANISM OF GENERATION OF OH radicals*
There are many reactions that generate hydroxyl radicals OH* based on oxidizing agents such as: Fenton, photofenton, electrochemical Fenton, peroxon, catazone, electrochemical oxidation, ultrasonic process, radiation process, high energy, UV/oxidation process, TiO2/UV semiconductor photocatalysis…
The mechanism of OH* generation depends on the type of AOT technique used. Common AOT techniques for wastewater treatment projects are assimilation Fenton (Fe2+/H2O2), catabolic Fenton (FeOOH/H2O2), Ozoneization (O3/H2O2), photocatalysis (TiO2). /UV)…
APPLICATION RANGE
AOP Oxidation technology is suitable for use in the following areas:
– Wastewater of electronic industries.
– Industrial & municipal wastewater.
– Wastewater Pharmaceutical & Biotechnology industry.
– Wastewater from oil and gas refineries.
– Chemical industry wastewater.
– Wastewater from power industries.
– Textile industry wastewater.
– Electroplating industry wastewater / metal finishing wastewater.
– Paper production wastewater.
– Wastewater leachate.
– Food and beverage wastewater.
Advantages AOP has outstanding advantages in the field of water treatment:
Effectively removes organic compounds in water, reacts with almost any contaminant in water
Removal of some heavy metals in the form of precipitates M(OH)x
Can be used in disinfection, as an integrated solution to many water quality problems.
AOP does not introduce any harmful substances into the water, the complete reduction products are OH and H2O.
Disadvantages of AOP
The biggest disadvantage of AOP is its high cost, as a constant input of expensive chemical reagents is needed to keep most AOP systems running. Due to their nature, AOPs require hydroxyl radicals and other reagents proportional to the amount of contaminant to be removed.
Some techniques require wastewater pre-treatment to ensure reliable performance, which can be costly and technical.
It is not effective to only use AOP to treat large amounts of wastewater; instead, AOP should be implemented at a late stage after primary and secondary treatment has successfully removed a large proportion of contaminants.


