SOLUTIONS TO TREAT AMONIUM AND PHOSPHORUS
Among the pollution indicators, ammonium and phosphorus pollution are also two important factors that need to be thoroughly treated. The discharge limits for ammonia are becoming more and more stringent, making ammonium and phosphorus treatment one of the most important and most difficult processes for wastewater treatment plants.

Impact of wastewater containing ammonium and phosphorus on the environment and people
Ammonium is a valence state of nitrogen, which exists in two forms, NH4+ ion and NH3 gas, accounting for 90-97% of total nitrogen. Ion NH4+ is less toxic, NH3 is a gas with a clear odor, colorless, highly soluble in water, causing death to organisms in the water. NH3 dominates when in high pH environment (pH ≈ 11), in low pH environment, NH4+ dominates (pH ≈ 7).
When present in high concentrations, ammonium is easily converted to form nitrite NO2- and nitrate NO3-. Through the process of eating, these substances will be absorbed into the body and cause a number of diseases
dangerous and transform into carcinogenic substances.
Phosphorus usually exists as soluble phosphates: orthophosphate, meta or polyphosphate and organic phosphate.
Ammonium along with untreated phosphorus, if discharged into ponds, lakes and canals, will become a source of nutrients for phytoplankton, moss, algae to thrive, causing eutrophication. Eutrophication reduces the concentration of dissolved oxygen in water, some toxic algae directly affect aquatic animals, can even cause death and back water pollution.
In the regulation QCVN 01:2009/BYT dated June 17, 2009 for drinking water, the concentration of ammonium in water is regulated.
should not exceed 3 mg/L.
In QCVN 40: 2011/BTNMT for industrial wastewater, the concentration of ammonium in treated wastewater should not exceed 5 mg/L. Phosphorus does not exceed 4 mg/l
Ammonia treatment method
Ammonia Treatment Facility
To treat ammonium in wastewater can use many methods such as physical, chemical or biological methods. The general principle of these methods is to convert ammonium into other substances or separate them from aqueous medium.
The selection of the appropriate treatment method is based on the ammonium concentration in the water as well as on the investment cost. If the ammonium concentration is not as high (<100 mg/l) as in domestic wastewater, the microbiological method is most appropriate, the ammonium concentration is from 100 to 5,000 mg/l, and the microbiological method is also used. evaporative aeration method can be used, ammonium concentration is greater than 5,000 mg/l, so using physico-chemical method will be suitable both technically and economically.
1- Physicochemical Method
Tripping controls pH: It is to raise the pH up to 11 to convert SMALL into NH. However, the efficiency is difficult to reach 80%.
Tripping temperature control: Provide heat for wastewater, treatment efficiency reaches 98%. However, this method consumes a large investment cost.
Ion exchange: Ammonium ions swap with cations in zeolites. Zeolite is a crystalline form of an aluminosilicate mineral with a porous anionic framework. This process is often depleted and zeolites often require frequent regeneration.
Zeolite NH4+ exchange process can be expressed by the formula: X + Z- + NH4 + → NH4 + Z- + X+
Where: Z represents anionic aluminosilicate, X represents ion exchange.
This process is often depleted and zeolites often require frequent regeneration.
2- Chemical Method
Chlorination method
Chlorination is considered to be the most effective ammonium reduction method as chlorine is almost the only chemical that can oxidize ammonium even at room temperature to produce volatile N2. The reaction rate of chlorine with ammonium is also faster than that of organic substances. The amount of chlorine added to wastewater compared to ammonium corresponds to a ratio of 8:1. This method is effective and cheap. However, it has the disadvantage that when the amount of ammonium is exhausted, the residual chlorine will react with organic substances in the water to create compounds with unpleasant odors, of which about 15% of substances belong to the THM group – trihalomethane and HAA – halogenated acetic acid are all potentially carcinogenic. Therefore, in the process of using the operator, it is necessary to have good technique and be very careful
Ozonation method
Using Ozone as a strong oxidizing agent to oxidize ammonium to N2, this method is safe and does not produce toxic byproducts such as chlorine. At the same time, Ozone is also used to disinfect, remove color, odor and other difficult-to-treat organic substances. However, the investment cost is high, and there are few applications for wastewater treatment.
3- Electrochemical method
To treat ammonium in wastewater, there are a number of studies that apply wastewater phase with 20% seawater and put into electrolysis tank with graphite anode and stainless steel cathode. Under the action of electric current, it will form magnesium hydroxide, which reacts with ammonium and phosphorus in wastewater to form an insoluble component, magnesium ammonium phosphate. In addition, the electrolysis process also forms Cl2 can ammonium oxygen, organic substances and bactericidal for wastewater.
The ammonium treatment efficiency of this method is 80-85%, the voltage used is about 7V, and the power consumption is at 200A/h for 1 m3 of wastewater.
The precipitate formed can be used as fertilizer.
See also: Electrochemical method
4- Biological Methods
Nitrification and denitrification processes

*Nitrification process
The oxidation of ammonium to nitrite (by nitrosomonas bacteria) and further to nitrate (nitrobacter bacteria) is directly dependent on the dissolved oxygen concentration, pH, temperature, etc. This process occurs in the aeration tank.
Step 1. Nitrosomonas bacteria will convert ammonium to nitrite
NH4+ + 1.5O2 → NO2– + 2H+ + H2O
Step 2. Nitrobacter bacteria will convert nitrite (NO2– ) to nitrate (NO3– )
NO2– + 0.5O2 → NO3–

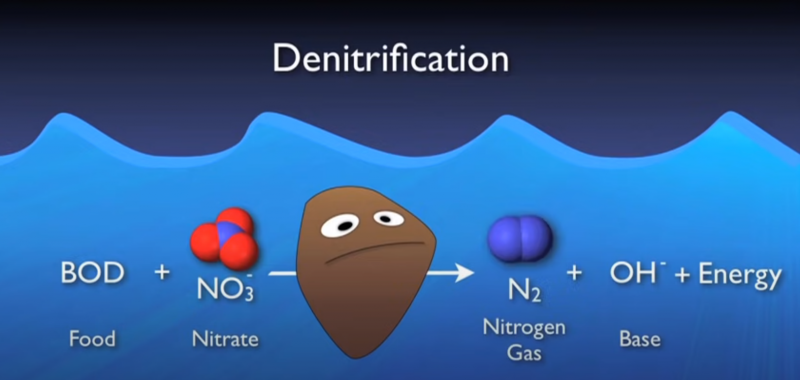
*Denitrification process
It is the process of microbial conversion of NO3–, NO2–, NO, N2O forms to N2 or the process of denitrification from the positive valence form to the negative valence form.
grandfather. In the anoxic environment, occurring in Anoxic tanks, these bacteria will reduce nitrate (NO3-) and nitrite (NO2-) according to the metabolic chain:
NO- → NO2- → NO → N2O → N2↑
The molecular nitrogen gas N2 formed will escape from the water and out. Thus the nitrogen has been treated. Microorganisms performing the nitrate reduction process have the common name Denitrifier including at least 14 types of microorganisms that can reduce nitrate such as Bacillus, Pseudomonas, Paracoccus, Spirillum, Thiobacillus, .. most of them are facultative, ie. is to use organic carbon sources to synthesize a few of the autotrophs.
Diagram depicting nitrifying bacteria
The key factor involved in the denitrification process is the substrate (organic matter, CH3OH), and controlling the oxygen concentration in the water for optimal denitrification efficiency.
In fact, technology engineers often apply synthetic works to combine BOD/Ammonium treatment in the same tank cluster.
A-O technology applies the nitrification / denitrification process to treat ammonium
AO technology stands for Anoxic (Anoxic) – Oxic (aerobic) is to use Anoxic tank cluster using anoxic bacteria to reduce nitrate to N2 and followed by Oxic tank containing aerobic bacteria that metabolize ammonium to nitrite and nitrate.

Image depicting AO
Nitrification happens first, denitrification happens later, so why are the tanks designed as A-O (anoxic first – aerobic later)
If aerobic treatment is done first, BOD can be completely lost, but N is only in the form of NO3-, not yet separated into the free form of N2. Then, the treated wastewater will meet the BOD and ammonium standards, but not the nitrate criteria.
Therefore, in the conventional design, to reduce the volume of the tanks, the Anoxic tank is designed before the Oxic tank, the conversion from NO3- to N2 takes place in the Anoxic tank thanks to the circulating pump.
Oxidation ditch technology
Oxidation ditch is one of the biological methods of wastewater treatment. And is an improved form of aerotank tank with agitation and extended aeration working mode. In order to thoroughly treat Ammonium Nitrogen in addition to organic matter treatment.
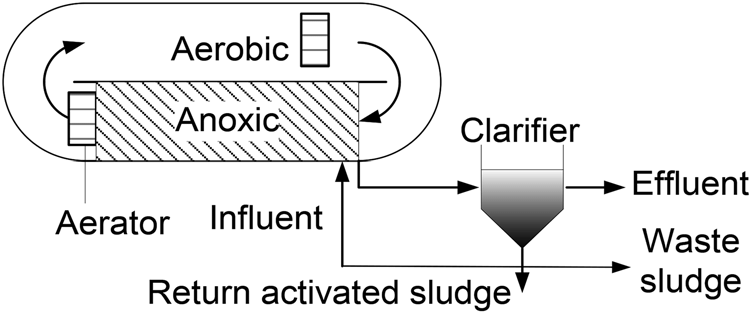
Figure depicting the oxidation ditch
Oxidation ditch has high BOD5 nitrogen treatment efficiency, easy to manage time, less affected by fluctuating inlet wastewater flow. Therefore, wastewater treatment areas with large construction areas are often prioritized to build oxidation ditch systems to optimize wastewater treatment efficiency.
PHOSPHORUS TREATMENT METHODS
PHOSPHORUS PROCESSING FACILITIES
Almost all phosphorus compounds do not exist in volatile form under normal conditions, so to remove phosphorus from water it is necessary to convert them to insoluble form before applying sediment separation techniques such as: : filter, settle or separate directly through a suitable membrane.
Phosphorus compounds in wastewater environment exist in the following forms: organic phosphorus, single phosphate (H2PO4-, HPO42-, PO43-) soluble in water, polyphosphate, also known as phosphate condensate, phosphate salts and intracellular phosphorus. biomass.
REMOVED PHOSPHORUS BY CHEMICAL METHODS
To remove phosphorus, one can use alum, iron salts or lime to chemically precipitate. This is considered the oldest method to reduce phosphorus dissolved in wastewater. Using alum or ferric salts is preferred because they work better at neutral pH of wastewater.
The disadvantage of this method is that the volume of sludge and metal salts in the sludge increases.
The process will completely remove phosphorus from wastewater by settling method.
Use aluminum alum to treat phosphorus according to the following chemical equation:
Al3+ + PO43+ AlPO4
Al3+ + 3OH– Al(OH)3
Use lime:
5Ca2+ + 3PO43- + OH– ↔ Ca5(PO4)3(OH)
Mg2+ + 2OH– Mg(OH)2
Ca2+ + CO32- CaCO3
Using iron alum:
Fe3+ + PO43- FePO4
Fe3+ + 3OH– Fe(OH)3
BIOLOGY METHODS
Biological phosphorus treatment can be achieved by circulating activated sludge under anaerobic and aerobic conditions. This process helps to form a population of microorganisms capable of storing phosphorus intracellularly in the form of polyphosphates, called polyphosphate-accumulating organisms (PAOs). If these PAO microorganisms exist in sufficient numbers, the Phosphorus will be removed along with the waste activated sludge.
The EBPR . process
In fact, when treating phosphorus by biological methods, an anaerobic process is applied when followed by an aerobic process. Also known as enhanced biological phosphorus removal (EBPR)
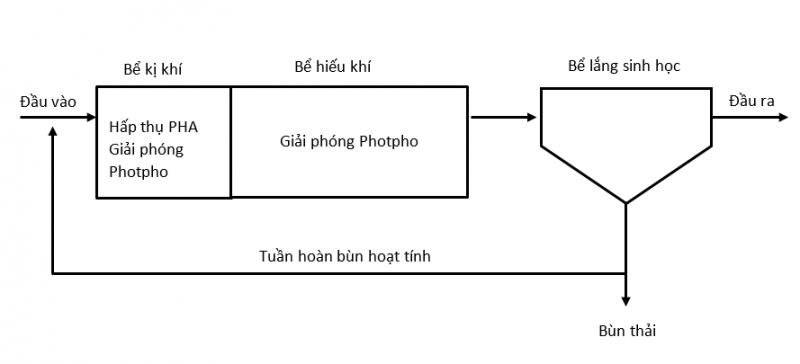
- Under anaerobic conditions, PAOS does not grow but converts simple organic materials such as volatile fatty acids (VFAs), mainly produced by microbial fermentation, into energy-rich polymers called polyhydroxyalkanoate (PHA) most commonly polyhydroxybutyrate (PHB) and polyhydroxyvalerate (PHV). At the same time, it releases a stored amount of orthophosphate inside the cell.
- Under aerobic conditions, PAO bacteria oxidize PHA and carbon source in wastewater as an energy source for biomass growth, demand for poly-P synthesis increases and glycogen is produced. The amount of poly-P accumulated in the biomass was as high as 15% of the dry weight. It is worth noting that more soluble phosphate is absorbed in the aerobic phase than is released in the anaerobic phase, leading to a decrease in P concentration in the wastewater. P is removed along with the amount of sludge after biological settling.
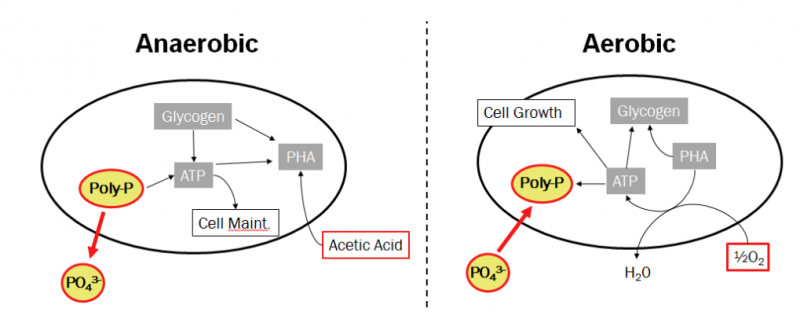
Microbial cells accumulate excess P in the biomass to form flocs that settle to the bottom of the settling tank, phosphorus contained in the sediment is removed and taken to a separate treatment, or reused as a fermentant to react with Volatile fatty acids increase the efficiency of phosphorus reduction.
Phostripe process
The PhoStrip process combines biological and chemical processes for phosphorus reduction. Most of the phosphorus in the circulating activated sludge is transferred to the anaerobic digester where the phosphorus is released in dissolved form. In the PhoStrip process, the phosphorus reduction efficiency is less dependent on the input rbCOD concentration than in other biological dephosphorylation processes.
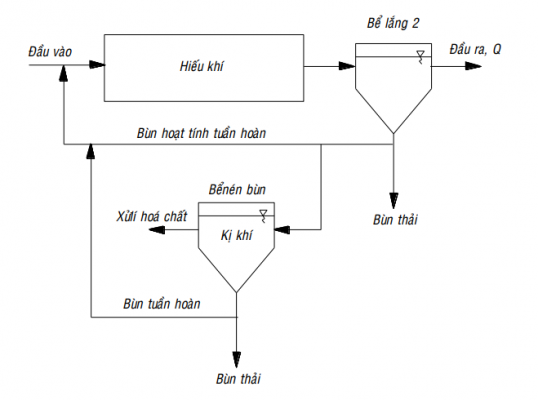
Image depicting PhoStrip
COMBINATION OF Ammonia and Phosphorus treatment by biological process
A2/O TECHNOLOGY
A2/O technology stands for Anerobic (anaerobic) – Anoxic (anoxic) – Oxic (aerobic). This technology is an improvement of the A/O process. Combining 3 micro-organisms: anaerobic, anaerobic, and continuous aerobic to increase the efficiency of ammonium and phosphorus treatment. Some PAOs have the ability to use oxygen in nitrate to absorb P and grow biomass, so in anoxic tank, besides N treatment, a significant amount of P can be reduced.
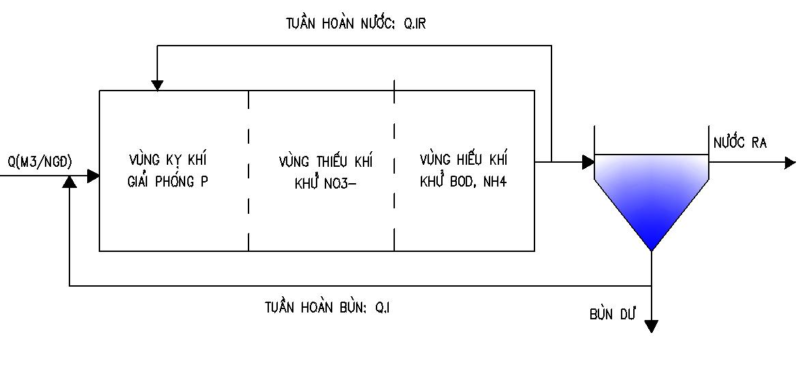
Figure depicting the A2O . model
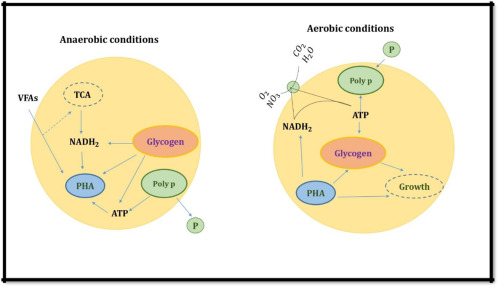
Figure depicting PAO accumulation in anaerobic and anoxic
UCT . process
The UCT process was created by the University of Cape Town. The UCT process was developed to minimize the impact of nitrates in substandard wastewater into the anaerobic contact zone. The amount of nitrate in the anaerobic zone determines the efficiency of biological phosphorus removal.
The UCT process for the A2/O process has two differences. The activated sludge is recirculated to the anaerobic stage instead of the aerobic stage, and the internal flow recirculates from the anaerobic to anaerobic stage. By circulating the activated sludge to the anaerobic stage, the introduction of nitrate to the anaerobic stage is eliminated, so that phosphorus uptake in the anaerobic stage is better. The characteristic of the endocirculatory flow is an increased use of organic matter in the anaerobic stage. The suspension from the anoxic stage contains a lot of dissolved BOD but little nitrate. The circulation of the anaerobic suspension provides optimal conditions for anaerobic fermentation.

Figure depicting the UCT . model
Bardenpho process (5 stages)
From the 4-stage Bardenpho tank to treat Nitrogen, add 1 more stage to combine the reduction of both nitrogen and phosphorus. Adding the 5th stage is the anarobic anaerobic process to reduce phosphorus on the first step of the combined process of reducing nitrogen and phosphorus. The arrangement of stages and the way of circulating the wastewater mixture after the zones are also different and different from the A2/O treatment process.
The 5-step system provides anaerobic, anoxic, aerobic zones to reduce both nitrogen, phosphorus and organic compounds. Anoxic zone (phase 2) for denitrification and supplemented with nitrate from the aerobic tank (phase 3). The last aerobic bath separates the N2 gas from the water and reduces the phosphorus content to a maximum. Processing time is longer than A2/O process. Total retention time is 10-40 days, increasing the biomass of microorganisms

Figure depicting the UCT . model
Bardenpho process (5 stages)
From the 4-stage Bardenpho tank to treat Nitrogen, add 1 more stage to combine the reduction of both nitrogen and phosphorus. Adding the 5th stage is the anarobic anaerobic process to reduce phosphorus on the first step of the combined process of reducing nitrogen and phosphorus. The arrangement of stages and the way of circulating the wastewater mixture after the zones are also different and different from the A2/O treatment process.
The 5-step system provides anaerobic, anoxic, aerobic zones to reduce both nitrogen, phosphorus and organic compounds. Anoxic zone (phase 2) for denitrification and supplemented with nitrate from the aerobic tank (phase 3). The last aerobic bath separates the N2 gas from the water and reduces the phosphorus content to a maximum. Processing time is longer than A2/O process. Total retention time is 10-40 days, increasing the biomass of microorganisms

Figure depicting struvite recovered in wastewater
Recent studies show that magnesium-ammonium-phosphate precipitation technology from wastewater to recover ammonium and phosphorus is not only cost-effective but also has great environmental significance.
Struvite is known as a slow-release fertilizer that provides both macro-elements (N: 6% and P2O5: 28.9%) and intermediates (Mg: 10%) suitable for many crops. Therefore, struvite is used as a raw material for the production of other mixed or complex fertilizers.
Thus, the precipitation of struvite and its derivatives from wastewater containing ammonium and phosphorus is considered an environmentally friendly treatment method that simultaneously removes both N and P from wastewater and obtains a product. valuable products for agricultural production.
MPTEK specializes in providing ammonium and phosphorus treatment solutions in industrial wastewater, domestic wastewater, urban wastewater… Liên hệ với chúng tôi ngay để được tư vấn và tìm giải pháp tốt nhất cho bạn!
MPTEKI SOLUTIONS CO., LTD
Hotline: 0903188563
Address: 20 Huynh Thien Loc, Hoa Thanh Ward, Tan Phu District, HCMC.