SOLUTIONS FOR HANDLING DIFFERENT ORGANIC CHARACTERISTICS
What is persistent organic matter?
Persistent Organic Pollutants or POPs are short for “Persistent Organic Pollutants”. POPs are groups of toxic, persistent and persistent organic substances in the environment with 4 main characteristics:
- (1) High toxicity
- (2) Hard to decompose in the natural environment
- (3) The ability to move and disperse far;
- (4) High bioaccumulation potential.
POPs are harmful to human health and the environment, including 22 groups of substances specified in the Stockholm Convention. Vietnam signed the STOCKHOLM Convention on May 23, 2001 and ratified it on July 22, 2002, and officially became the 14th member of the Convention.
Impact of POPs on humans and the environment
Persistent organic compounds POPs are typical hazardous wastes with low water solubility, high accumulation potential, and carcinogenic, teratogenic and neurotoxic properties. For example, Dioxins and benzofurans are highly toxic and extremely persistent in the human body as well as the environment. Several POPs, including DDT and its metabolites (PCBs, dioxins and some chlorobenzenes) have been detected in human body fat. Lindane (hexachlorocyclohexane) is a broad-spectrum insecticide that can cause acute death when used improperly.
Types of wastewater containing POPs and treatment methods.
Types of wastewater often contain POPs
- Leachate
- Agricultural wastewater.
- Wastewater from chemical industry, pesticide and pesticide production.
- Electronic industry wastewater (transformers, capacitors)
- Textile dyeing wastewater…
There have been many reports of various advanced technologies succeeding in POP treatment, but in reality there are still many obstacles such as: low decomposition efficiency, generation of toxic intermediates, large amount of sludge. , energy consumption and high operating costs. However, advanced oxidation processes (AOPs) have recently recorded success in the treatment of POPs in wastewater. AOPs is a technology involving generation of hydroxyl radicals OH*, which aim to oxidize persistent organic contaminants to their inert end products which can even mineralize to CO2+ H2O.
See also: Advanced Oxidation Processes (AOPs)
To treat toxic or non-biodegradable organic matter with not too high concentrations in wastewater, the AOP method can be used. In fact, many substances that are difficult to biodegrade are also difficult to oxidize, so strong oxidizing agents are required. The oxidizing capacity of oxidizing agents is assessed through the redox potential, the higher the redox potential, the stronger the oxidizing agent. Among the oxidizing agents, the radical OH* is the strongest, only inferior to fluorine. In addition to the oxidation potential, the reaction rate must also be taken into account. The reaction rates are compared through the rate constant k. The k constant of the reactions between OH* radicals with organic substances is very high, millions to billions of times higher than that of ozone. With the above advantages Advanced oxidation process is considered as the optimal solution to treat persistent organic matter in wastewater. MPTEK introduces some AOPs commonly encountered in wastewater treatment systems containing persistent organic matter.
1. FENTON PROCESS
The Fenton process has been known since 1894 with a combination of H2O2 and Fe2+ iron salts used as oxidizing agents for many wastewater containing difficult-to-treat pollutants. Since it is known, in the world, there have been many studies applying the Fenton process to the treatment of many types of wastewater such as textile wastewater, paper wastewater, oil refining wastewater, toxic chemical industries, etc. positive results. Fenton oxidation usually has 4 stages:
Adjust pH accordingly: Create an acidic environment: 2 < pH < 5 (for homogenous fenton process)
- Oxidation reaction
During the oxidation reaction phase occurs the formation of active •OH radicals and the oxidation reaction of organic substances. The process follows the following reaction Fe2+ + H2O2 → Fe3+ + OH* + OH- The above reaction is called the Fenton reaction because he was the first to describe this process. The OH* radical, after formation, will participate in the oxidation reaction of organic compounds in the water to be treated: converting organic matter from high molecular weight to low molecular weight CHC (high molecular weight) organic matter. atom) + OH* → CHC (low molecular weight) +CO2 +H2O+ OH-
See also: Fenton process in wastewater treatment
- Neutralization and Coagulation
After the oxidation occurs, it is necessary to raise the pH of the solution to >7 to make the newly formed Fe3+ precipitate: Fe3+ + 3OH- → Fe(OH)3 ↓ The newly formed Fe(OH)3 precipitate will be formed. Mechanisms of coagulation, coagulation and partial adsorption of organic substances are mainly macromolecular organic substances.
- Settling process:
After forming, the colloidal cotton will settle, reducing COD, color, and odor in wastewater. After settling, the remaining organic substances (if any) in wastewater are mainly organic compounds with low molecular weight, which will be additionally treated by biological methods or by other methods.
2. ADVANCED OZONE-BASED OXIDATION PROCESS
Ozone (O3) is a strong oxidizing agent among common oxidants, with an oxidation potential of 2.07V. Ozone can oxidize different substances in two ways:
- Direct oxidation by ozone molecules dissolved in water;
O3 + R → SP
- Oxidation via hydroxyl radical OH● is produced by the decomposition of ozone when dissolved in water.
O3 → OH* OH* + R → SP
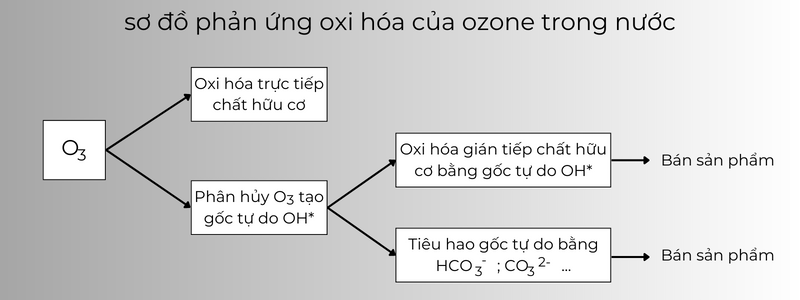
Direct oxidation by O3 molecule occurs relatively slowly compared to indirect oxidation via hydroxyl radical due to ozone decomposition. In acidic environment, direct oxidation by O3 molecule is predominant. The O3 molecule reacts readily with organic substances such as amines, phenols and aromatic compounds, but reacts slowly with carboxylic acids, aldehydes and alcohols. In an alkaline environment, in the presence of OH– ions, O3 is rapidly decomposed and forms OH* radicals, which oxidize organic substances in water and wastewater. The reaction of molecular O3 (E0 = 2.07V) is slow and limited while the radical OH* (E0 = 2.80V) reacts with most organic substances in water and wastewater. The reaction of three O3 molecules to form two OH* radicals:
3O3 + OH- + H+ → 2OH* + 4O2
Therefore, instead of using Ozone, many research works have developed towards searching for agents that combine with ozone or catalysts to generate OH* radicals to improve the oxidizing efficiency of ozone when it is necessary to treat ozone. management of stable, non-biodegradable compounds in water and wastewater. The most studied adding agents are H2O2, catalysts are salts of Ni(II), Co(II), metal oxides TiO2, MnO2…
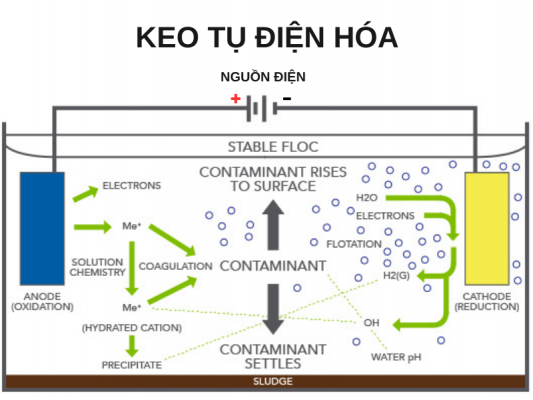
3. EC/EO ELECTRONIC METHOD
- Electrolytic condenser (EC)
Electrochemical coagulation is often known as a method to improve the traditional physico-chemical treatment process, but in addition to the flocculation process that precipitates pollutants, EC also performs oxidation. 1 part non-biodegradable pollutant. In fact, depending on the operation and type of water to be treated EC can oxidize dissolved COD with efficiency up to 95% or even more.
- Electrochemical Oxidation (EO)
Electrochemical oxidation is an optimal solution for the rapid decomposition of persistent organic substances such as aromatic, chlorinated, phenolic organic compounds. This is a very good solution to support the biological treatment process as well as an alternative solution for cases where biological oxidation cannot be applied.
See also: Electrolysis technology (EC/EO)
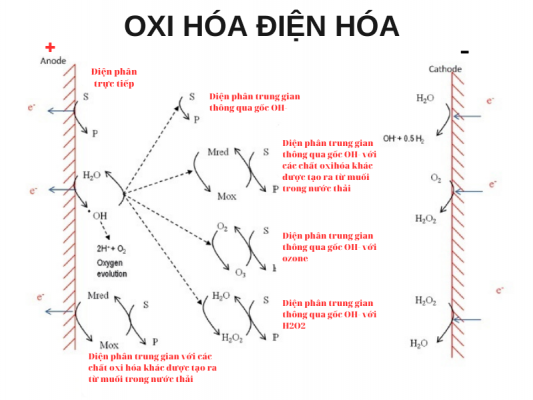
In EO, oxidation of pollutants takes place directly on the anode surface through the in situ generation of hydroxyl radicals (OH*) or metal oxides (MO) from salts in the electrolyte solution. The OH* radical (Eo(OH/H2O) = 2.8 V) is generated in situ as an intermediate in the electrolysis of O2 gas from water molecules on the anode surface (1). For active electrodes, there is a strong interaction between the electrode (M) and OH, leading to the formation of higher metal oxides (MO)(2). Both substances are physically adsorbed with the *OH radical (formed on inert electrodes) and chemically adsorbed with MO metal oxide (formed on active electrodes) reacting with organics. until the organic matter is fully/partially mineralized (3) (4). These organic oxidation reactions take place simultaneously with the side reactions of O2 electrolysis without any involvement of the anode surface. M + H 2 O → M ( *OH) + H+ + e – (1) M ( *OH) → MO + H+ + e – (2) M ( *OH) + R → M + mCO 2 + n H 2 O + H+ + e – (3) MO + R → M + RO (4) 2H 2 O → O 2 + 4 H + + 4 e − (5) Where, R is the organic compound/pollution and M (*OH) is the hydroxyl radical adsorbed on the anode (M) or remaining near the anode surface. MOs are higher metal oxides formed on the anode through reaction with hydroxyl radical.
4. NANO PROCESSING TECHNOLOGY
Nanoprocessing technology for POP removal with a particular focus on nanocatalysis, nanofiltration and nanoadsorption processes. Nanoparticles such as clay, zinc oxide, iron oxide, aluminum oxide and their composites have been widely used for efficient treatment of POPs.
From some literature, it has been found that nanofiltration based techniques have shown complete removal of POPs from wastewater compared to conventional methods, but cost is one of the main issues when it comes to filtration. nano and ultrafiltration. Future research on nano-based techniques for POP remediation will address the cost issue and will make it one of the most widely available and accepted techniques.
NANO FILTER (TYPES OF NANO FILTER)
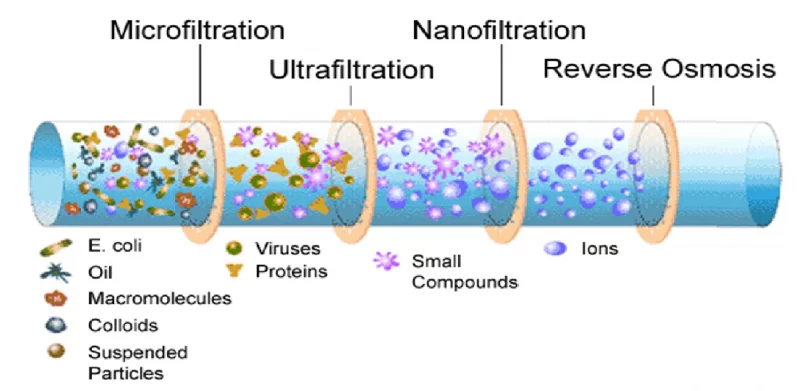
Nanotechnology has paved the way for advanced water treatment systems using nanofiltration membranes. Membrane processes such as microfiltration (MF), reverse osmosis (RO), ultrafiltration (UF) and nanofiltration (NF) are pressure-controlled filtration techniques and are considered highly efficient processes to wastewater treatment. They are considered as alternative methods to treat trace organic pollutants present in water.
Although the treatment of wastewater by membrane processes is expensive, they are the best alternative to conventional techniques because of their very high removal efficiency. Nanoparticles can be incorporated into membranes by surface immobilization, blending or grafting to the surface to grow the film depending on the desired function and properties. By modern methods, polymeric or composite nanofiber membranes can be developed to synthesize ultrafine nanofibers using materials such as Ceramic, polymer or metal in the 10–1000 nm range.
Among all membrane techniques, NF and RO have demonstrated their effectiveness in the treatment of trace organic pollutants. NF is relatively more effective at treating pollutants than RO (disadvantage is high energy consumption and maintenance costs).
NF is effective in the treatment of micro-pollutants due to its small pore size, high efficiency and user-friendliness. Several polymers (natural and synthetic) have been used to prepare nanofiltration membranes such as polypropylene, polyvinyl fluoride, polyacrylonitrile and most commonly cellulose acetate, as they are effective in removing POPs. Nanofibers have a stable adsorption structure compared to nanotubes and nanoparticles. Nanofibers have been shown to be effective in removing pesticides from wastewater through their molecular propagation mechanism; Furthermore, when semiconductor materials are used to synthesize nanofibers, they can add photocatalytic properties. Some nanocomposite nanofiber films (ZnO-cellulose acetate, TiO 2 -graphene, etc.) exhibit strong photocatalytic efficiency for the treatment of colored compounds.
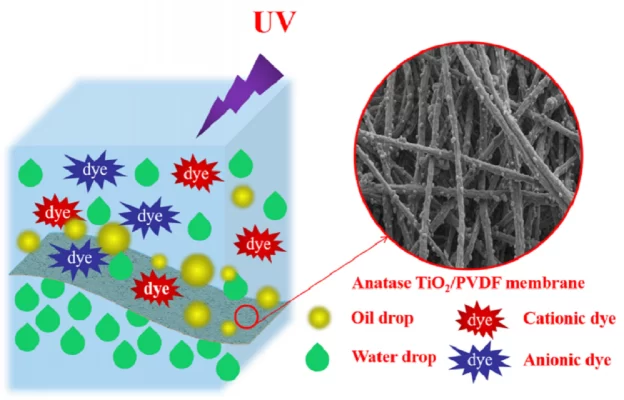
Nanofiltration is a hydrodynamic-based pressure control technique between the membrane surface and membrane nanopores and is effective in processing low-molecular-weight compounds with a size range of 1– 10 nm.
On the efficient removal of atrazine and diazinon from wastewater using a synthetic polyamide nanofiltration membrane synthesized through condensation. The results showed that the water permeability and removal of diazinon was 95.2% for the unmodified film and 98.8% for the modified film showing a significant improvement in the performance of the poly(piperazine amide) film. TFC NF for pesticide removal. (Research conducted by Karimi et al. 2016)
Membrane filtration is considered the safest technology and NF is an excellent method to remove low molecular weight compounds. NF is the only filtration technology known to successfully remove pesticides and other organic pollutants. However, membrane clogging is a drawback of filtration technology, which can be overcome by using a combination of other technologies to filter MF, UF, etc.
NANO CATEGORY
The use of wideband semiconductor nanomaterials to process pollutants into environmentally friendly compounds is realized in the form of nanocatalysts. Semiconductor metal and metal oxide nanomaterials have received considerable attention in the sustainable treatment of POPs. Several types of nanocatalysts are used to efficiently degrade POPs from wastewater such as Fenton-based catalysts, electrochemical catalysts, photocatalysts and even multifunctional combinations of nanocatalysts. Photocatalyst/nanocatalysis is a well-known AOP; it is used to enhance the biodegradability of POPs by using strong reactive oxygen species (ROS) to react with organic pollutants.
Photocatalysis involves catalytic activation in the presence of light and relies on the generation of ROSs, i.e. radicals (hydrogen peroxide) H2O2, (superoxide anion) O2, (Ozone) O3 and (hydroxyl) radical) OH* destroys almost all organic molecules. Photocatalysis is even effective for the treatment of volatile organic compounds (VOCs) such as PCBs, Dioxins and PHAs by generating free radicals. The photocatalysis starts from a nanocatalyst with a wide band gap (such as ZnO, TiO 2 , WO 3) which becomes photoexcited when light source (natural or artificial) and oxygen are used for the separation. cancel POP. The ideal photocatalytic decomposition process includes the following steps, as shown in Fig
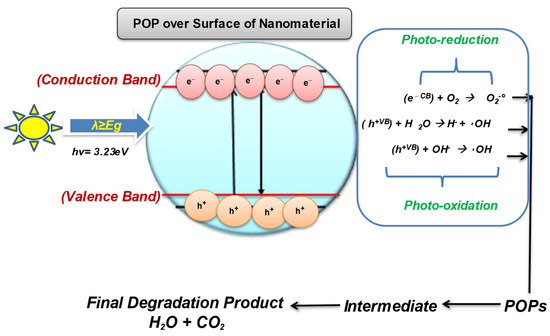
Up to now, TiO2 and ZnO are the most widely used semiconductors for POP decomposition. Of these, titanium dioxide (TiO2) is said to be the most effective. TiO2 has a very strong photocatalyst with strong oxidizing and decomposing ability of impurities on the catalyst surface.
See also: TiO2 .-based photocatalysis
NANO SUBTITLE
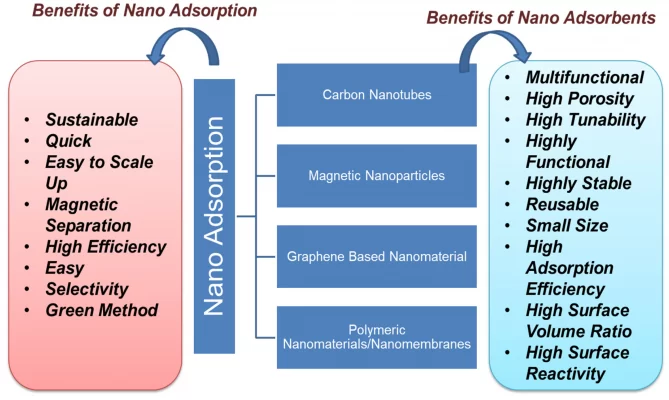
The nanoadsorbents offer high absorption efficiency due to their extremely large surface area and absorption site, adjustable pore size, much lower inter-particle diffusion distance and surface activity. high to effectively adsorb a variety of organic pollutants and pollutants. The advantage of using nano-adsorbents is that they can be easily functionalized to make them highly selective for any given pollutant. The adsorption process has been shown to be successful in the treatment of POPs such as hydrocarbons, dyes, phenols, detergents, pharmaceuticals, pesticides and biphenyls.
The figure depicted below shows different types of carbon-based nano-adsorbents and their benefits.
Nanoadsorption is an easy and safe process to treat POPs in water. Among the various technologies, nanoadsorption has so far emerged as a widely effective method for POP remediation.
Several studies demonstrate the effectiveness of nanomaterials in adsorbing various POPs from wastewater, as removal efficiency of more than 90% was achieved in most studies for up to 10 cycles. The adsorption efficiency of nanomaterials was mainly monitored by forming a complex with metal oxide surface and maintaining electron oxidation reaction under visible radiation. Nanoadsorption is based on electrostatic interactions, hydrogen bonding and hydrophobic interactions such as van der Waals, electron acceptor, etc.
Nanomaterials such as clay, zeolite, aluminum oxide, metal/metal oxide, activated carbon, carbon-based nanomaterials, composite nanomaterials, nanosheets, nanotubes, chitosan-based polymers and materials Graphene-based nanoparticles are widely used in nano-adsorption. For the efficient removal of POPs, the use of magnetic nanoparticles, especially iron oxides, has led to a new type of magnetic separation strategy.
The microporous structure present in activated carbon supports effective adsorption in removing POPs. Carbon-based nano-adsorbents tend to interact with contaminants due to hydrophobicity, hydrogen bonding, and covalent and electrostatic interactions. Each form has several adsorption sites that can adsorb organic pollutants due to their versatility. Both single-walled and multi-walled carbon nanotubes have been surface-modified by increasing porosity to create high-energy sites to absorb many organic pollutants.
Although nano application methods in POP treatment are still in the experimental stage, some have advanced to pilot scale. Therefore, nano-based technology is considered to have revolutionized the field of wastewater treatment.
Nguồn tham khảo: Nanotechnological Approaches for Removal of Persistent Organic Pollutants
MPTEK specializes in providing solutions to treat persistent organic matter in wastewater and feed water with advanced and modern technology.
Contact us now for advice and find the best solution for you!
MPTEKI SOLUTIONS CO., LTD
Hotline: 0903188563
Address: 20 Huynh Thien Loc, Hoa Thanh Ward, Tan Phu District, HCMC.